Investigation of structure-property-relationships
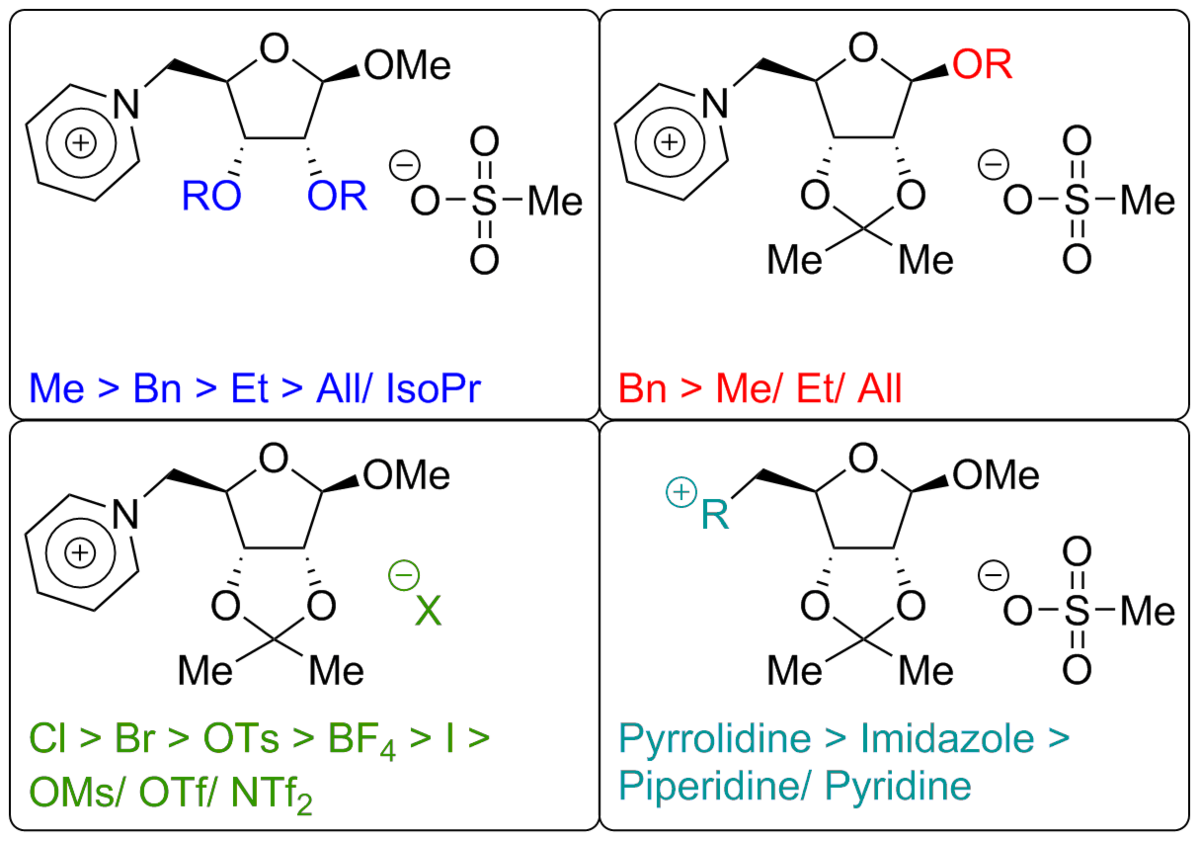
The combination of cations and anions with low interactions leads to ionic liquids - salts with melting points lower than 100 °C. Commonly used cationic N-heterocycles like imidazole in combination with anions like tetrafluoroborate, triflate or bistriflylimide are known to readily yield ionic liquids. But what happens when you use a cationic or anionic carbohydrate? We investigate the influence of protecting groups, anomeric variations and the carbohydrate itself on the properties of CHILS!
Biocompatibility and Biodegradability of Carbohydrate-based Ionic Liquids and Salts
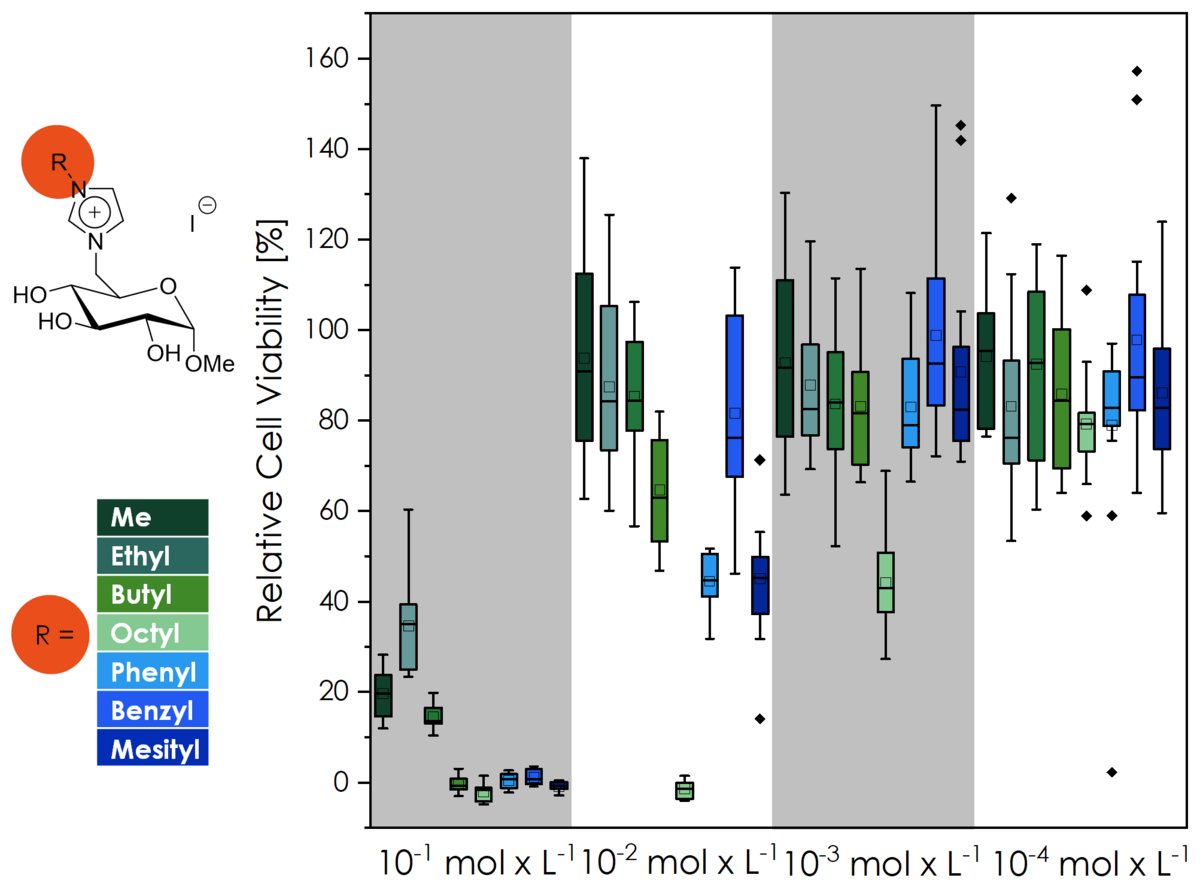
Ionic liquids have long been known as "green solvents", owing this name to their non-volatility and non-flammability. Commonly used ionic liquids like [BMIM][NTf2] are however toxic substances whose environmental long-term effect is barely known. Ionic liquid-like molecules sourced from natural resources on the other hand are known to exhibit a higher biocompatibility and better biodegradibility. Our group were the first to ever investigate the biocompatibility of CHILS (via the cell viability of L929 mouse fibroblasts)! This effort is ongoing, and we are ever trying to further improve the biocompatibility and are planning to investigate the biodegradibility of CHILS in the future as well.
Polymers and Hydrogels from CHILS
Hydrogels are three-dimensional polymer networks that swell but do not dissolve in water. They are either prepared by polymerizing water-soluble monomers in the presence of a crosslinker or by crosslinking an already existing (bio)polymer. Hydrogels are commonly used as superabsorbers (e.g. in diapers), in wound-dressing or as water-reservoir in agriculture among many other applications. An often researched aspect of hydrogels is their ability to intake other water-soluable substances together with water, thus allowing a slow emission of said substance from the hydrogel over time. We are investigating Glucosyl Vinyl Imidazolium (GVIM)-based monomers for the synthesis of hydrogels and subsequently their swelling degree, rheological properties, biocompatibility and more.
CHILS in Biocatalysis
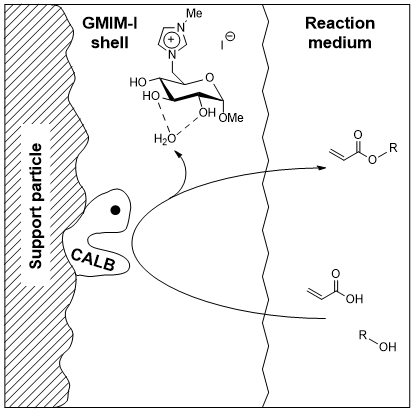
Biocatalysis offers an attractive option in the organic chemists toolbox for highly chemo- and stereoselective reactions. At the same time, ambient reaction conditions can be realized if enzymes are used as catalysts, which is a key point with the green chemistry aspect in mind. Our CHILS can be used to further enhance the catalytic activity of commercially available biocatalysts like Novozym 435 when adsorbed onto the catalyst surface via SILP (supported ionic liquids phase). We investigate the influence of the CHILS structure on the biocatalytic activity in the synthesis of industrially relevant building blocks!
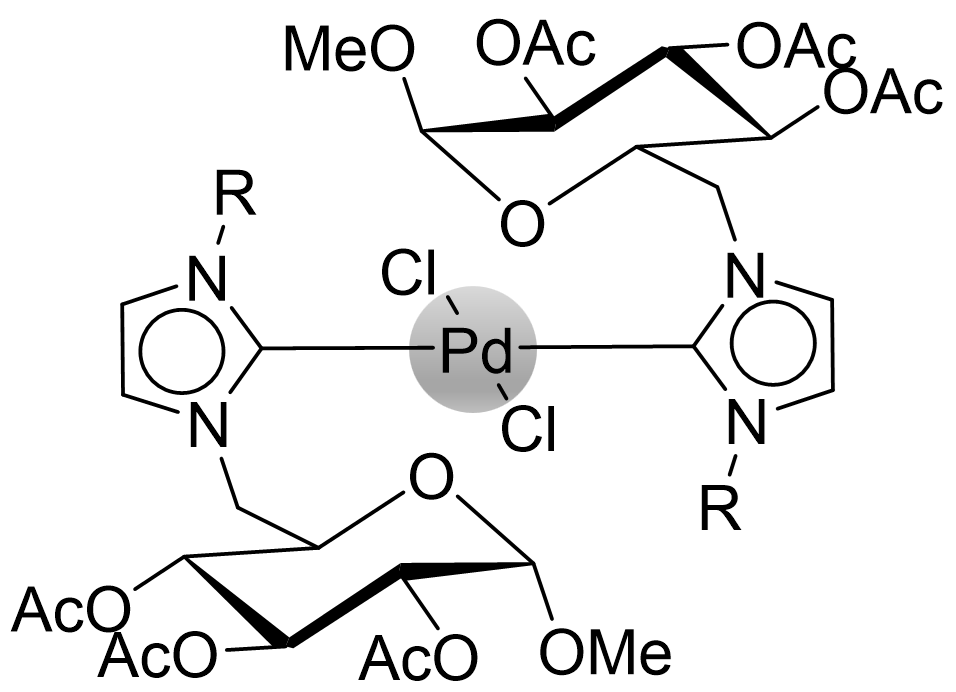
Organometallic Complexes from CHILS
Imidazolium based ionic liquids are well known in literature as precursors for N-heterocyclic carbenes (NHCs). These molecules are stable carbenes, where the electron-pair is located at the NCN-bridge of the imidazoylidene. NHCs are often used as ligands for metals and organometallic complexes containing NHC ligands are usually very "bench-stable". CHILS containing a cationic imidazolium ring may also be used as precursors for NHC complexes, which leads to carbohydrate-containing organometallic complexes, which are interesting due to expected lower toxicity and higher water solubility. Our group has so far investigated our CHILS for Pd-complexes, which where found to be highly reactive in Suzuki-Miyaura cross-coupling reactions.