Publications
26. M. Komabayashi, S. Jopp*, ChemistryEurope 2024, 2, e202400052.
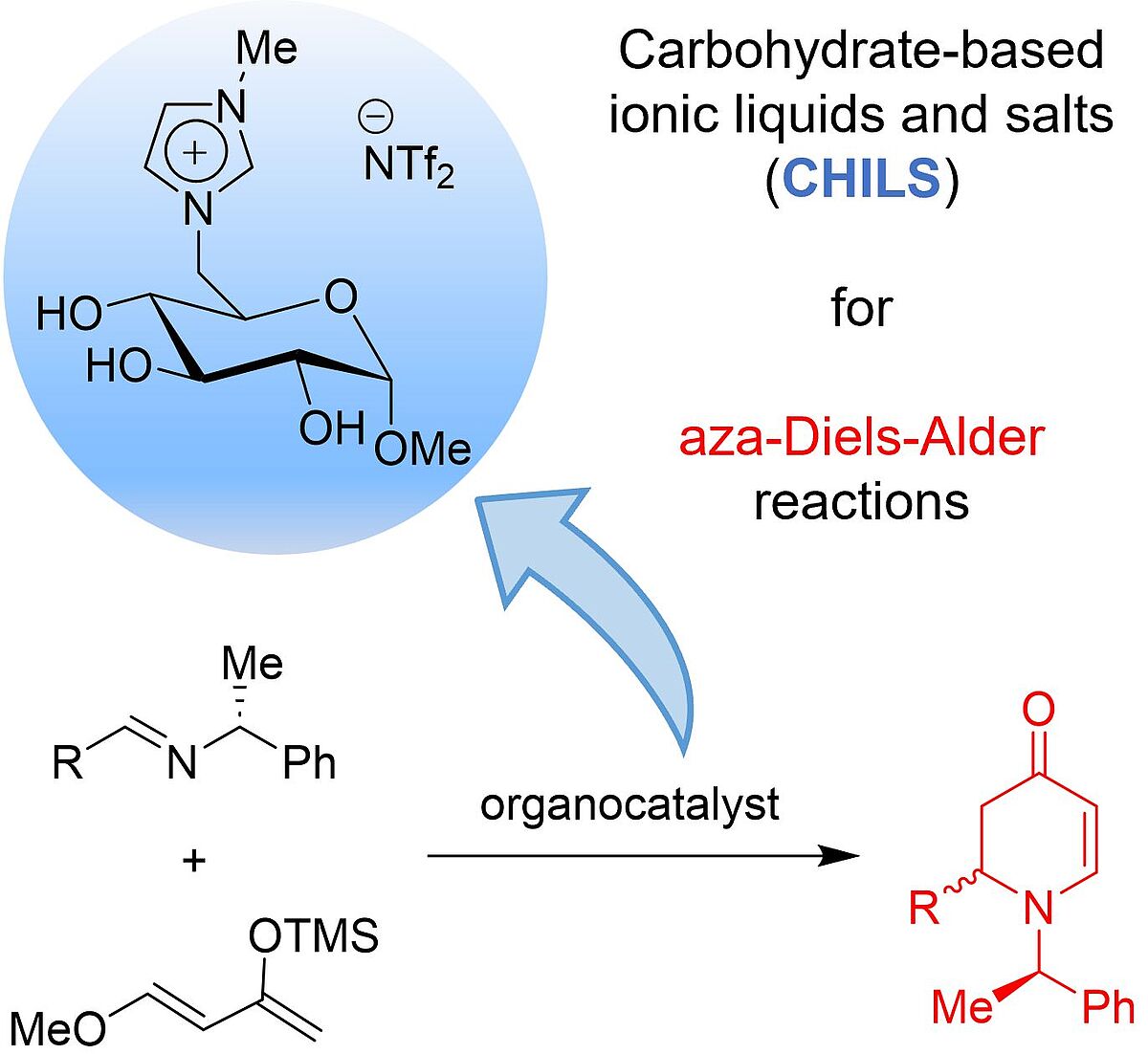
Glucose-based Ionic Liquid Organocatalysts for Asymmetric aza-Diels-Alder Reactions (Open Access)
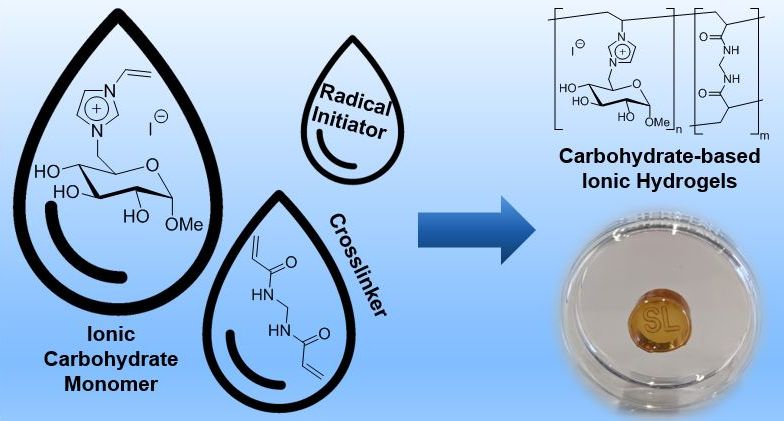
25. S. Lambrecht, A. Gazizova, S. Kara, J. Meyer*, S. Jopp*, RSC Adv. 2024, 14, 30719–30731.
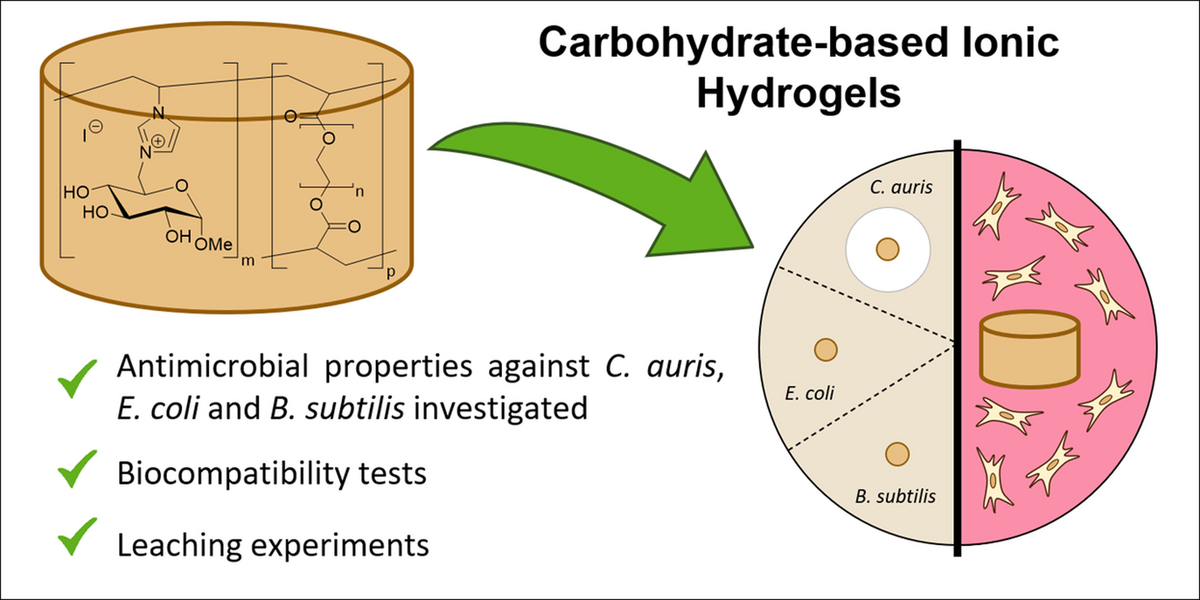
Antimicrobial properties and biocompatibility of semi-synthetic carbohydrate-based ionic hydrogels (Open Access)
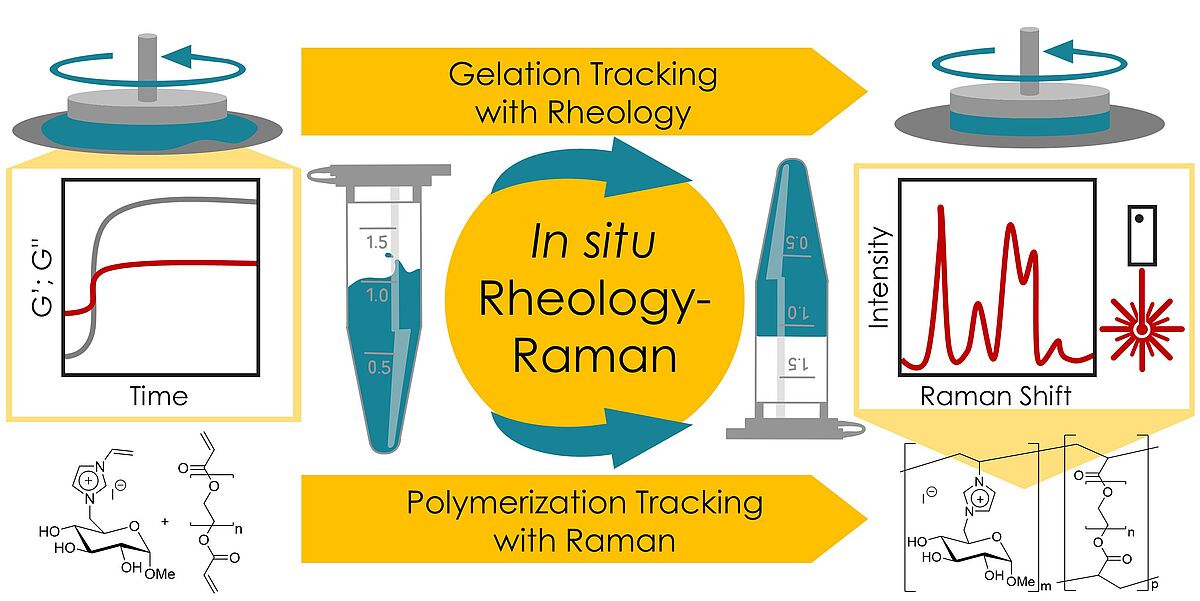
24. S. Lambrecht, M. Biermann, S. Kara, S. Jopp, J. Meyer*, Mater. Adv. 2024, 5, 6957–6966.
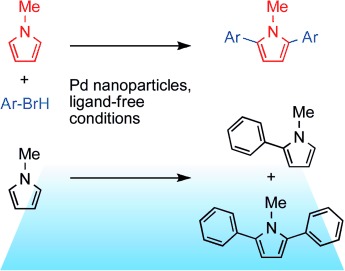
23. S. Jopp, F. Spruner von Mertz, P. Ehlers, A. Villinger, P. Langer*, Beilstein J. Org. Chem. 2024, 20, 1246–1255.
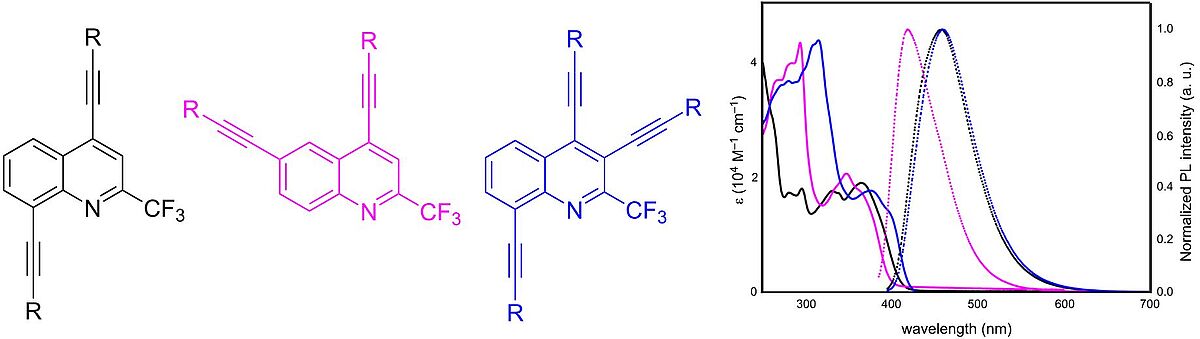
Synthesis and optical properties of bis- and tris-alkynyl-2-trifluoromethylquinolines (Open Access)
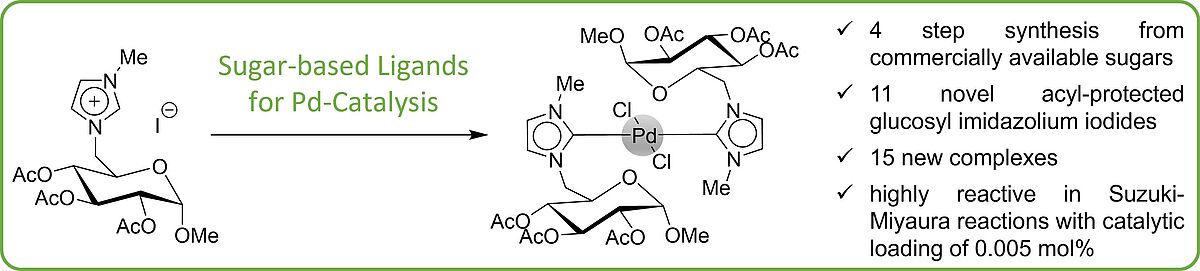
22. H. Ziems, M. Komabayashi, P. Lehmann, A. Villinger, S. Jopp*, Eur. J. Org. Chem. 2024, 27, e202400177.
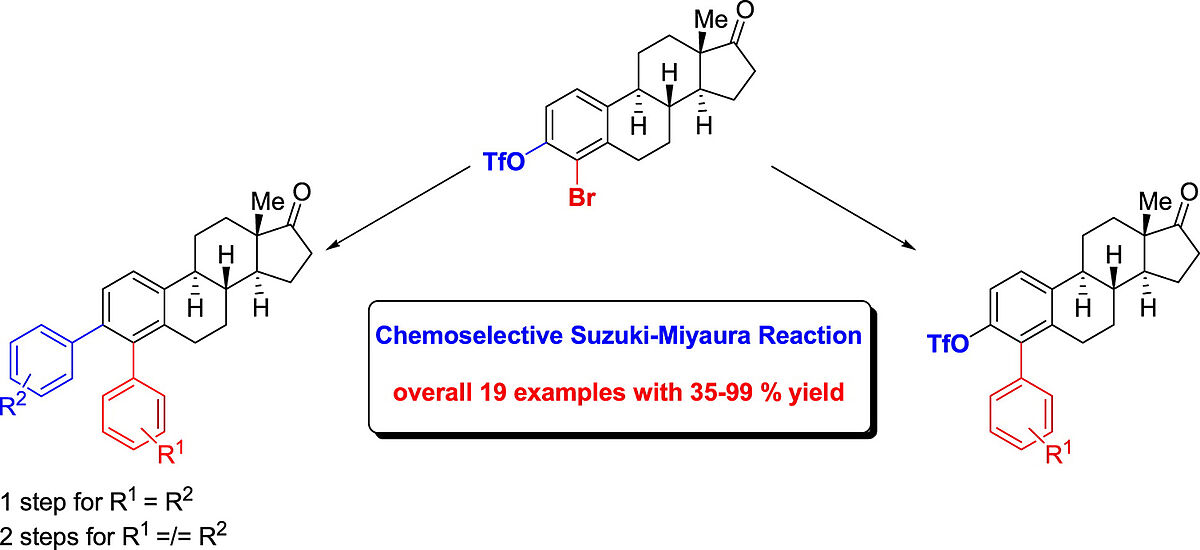
Structure-Activity Relationships of Glucose-based PdII-Bis(NHC) Complexes in a Model Suzuki-Miyaura Reaction (Open Access)
21. S. Lambrecht, H. Schröter, H. Pohle, S. Jopp*, ACS Omega 2024, 9, 5418–5428.
Swelling Behavior of Novel Hydrogels Produced from Glucose-Based Ionic Monomers with Varying Cross-Linkers (Open Access)
20. P. Lehmann, S. Jopp*, Chem. Asian J. 2024, 19, e202300918.
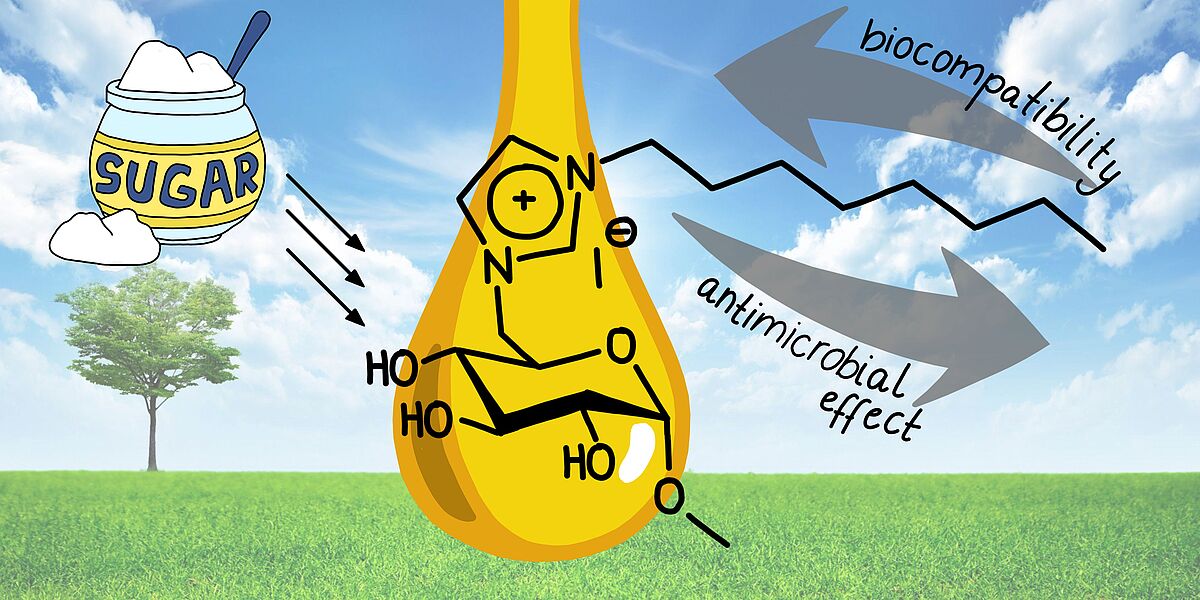
19. S. Jopp*, T. Fleischhammer, A. Lavrentieva, S. Kara, J. Meyer*, RSC Sustain. 2023, 1, 1751–1764.
Synthesis, biocompatibility, and antimicrobial properties of glucose-based ionic liquids (Open Access) + Backcover
18. M. Komabayashi, S. Okushiba, T. Nokami, S. Jopp*, Asian J. Org. Chem. 2023, 12, e202300093.
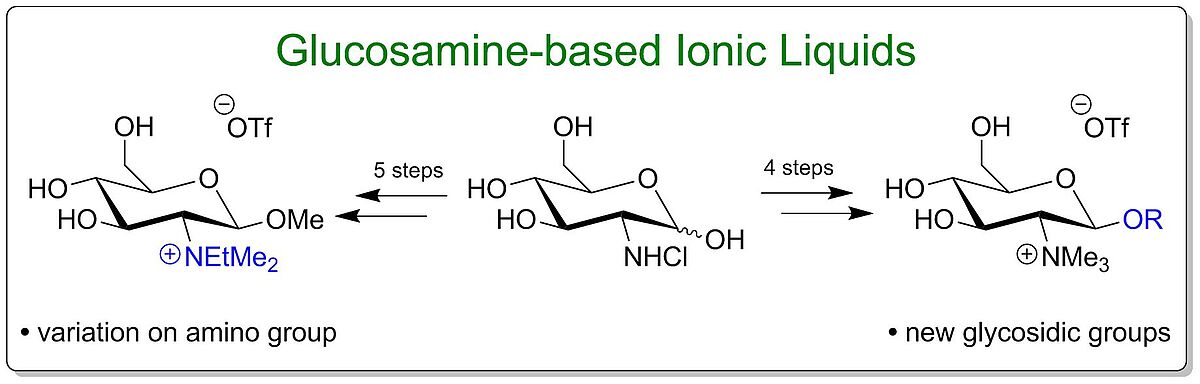
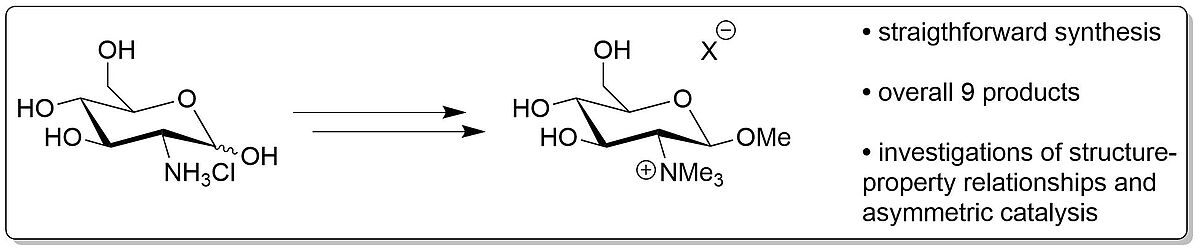
Scope and Limitations in the Synthesis of Glucosamine-based Ionic Liquids (Open Access)
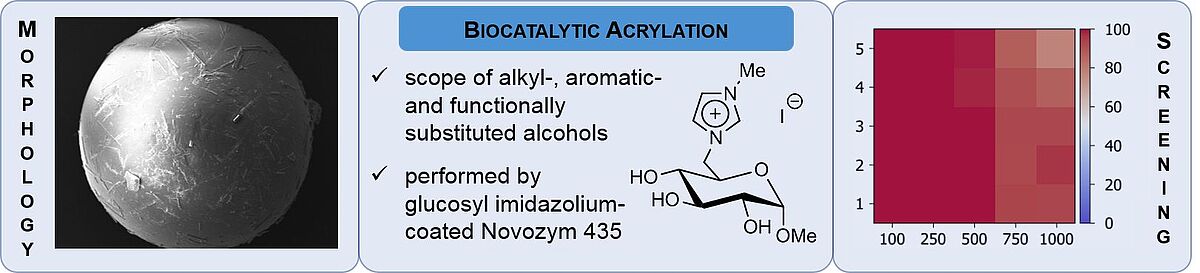
17. P. Lehmann, S. Jopp*, ChemistryOpen 2022, 11, e202200135.
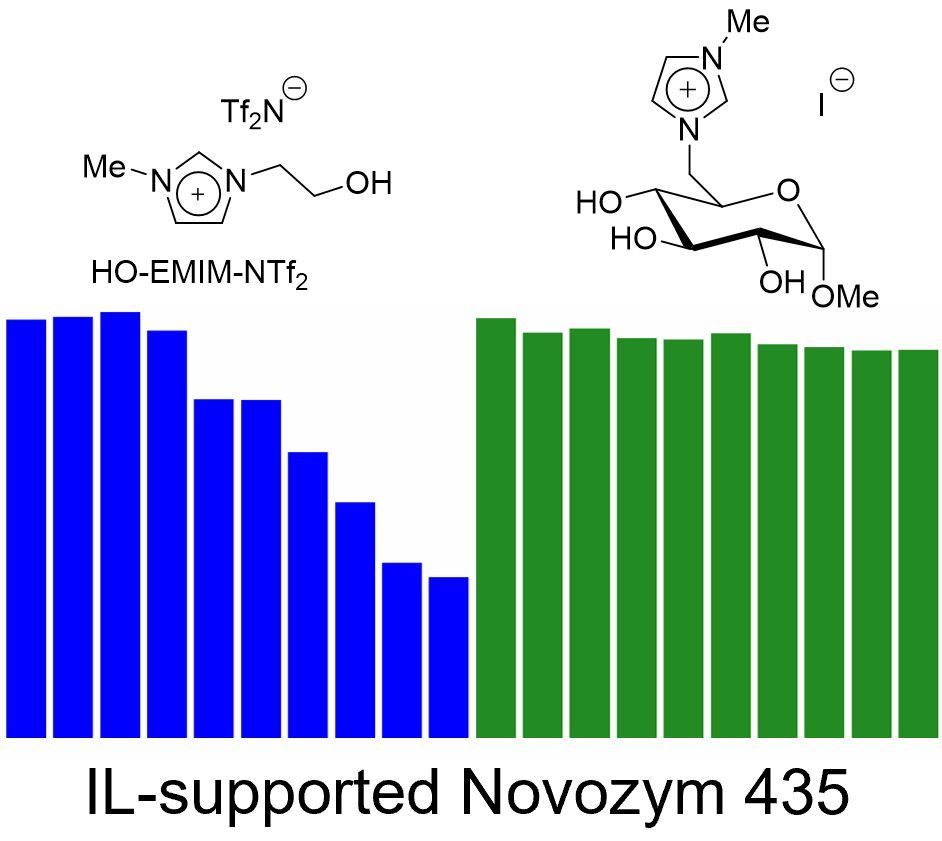
Novel Glucosylimidazolium Ionic-Liquid-Supported Novozym 435 Catalysts – A Proof of Concept for an Acrylation Reaction (Open Access) + Frontcover + Cover Profile
16. S. Lambrecht, A. Villinger, S. Jopp*, IUCrData 2022, 7, x220265.
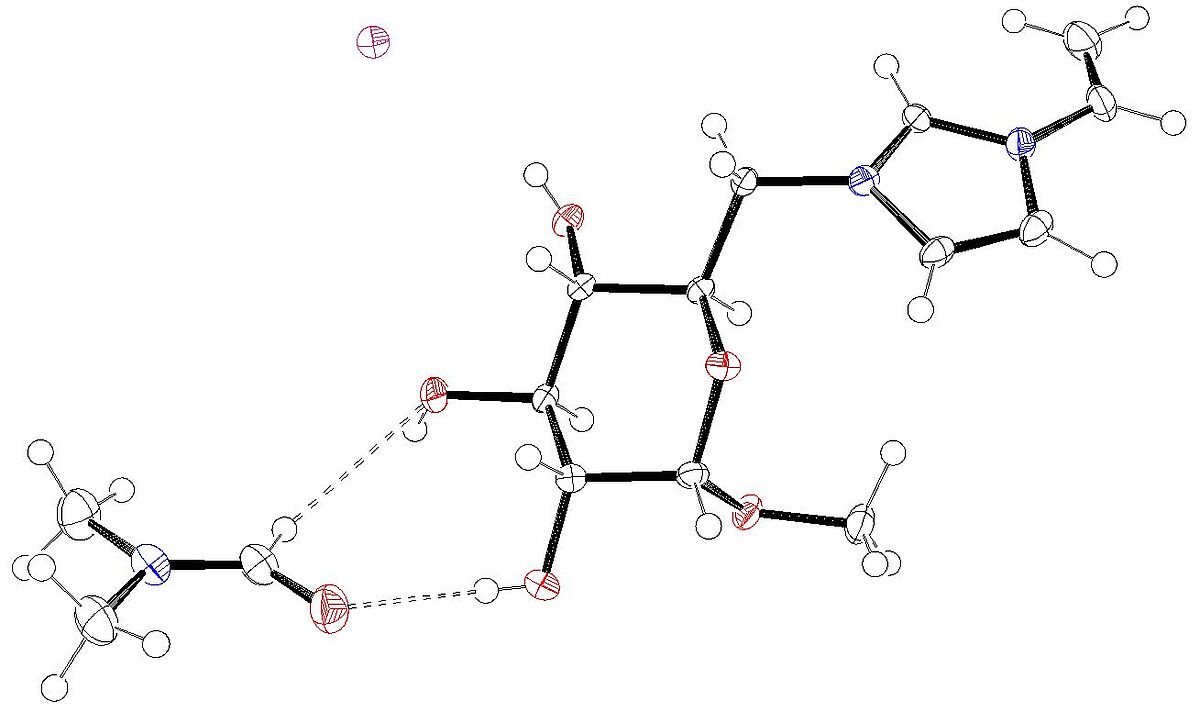
1-(Methyl-α-D-glucopyranosid-6-yl)-3-vinylimidazolium iodide dimethylformamide monosolvate (Open Access)
15. J. Schnegas, S. Jopp*, Compounds 2021, 1, 154–163.
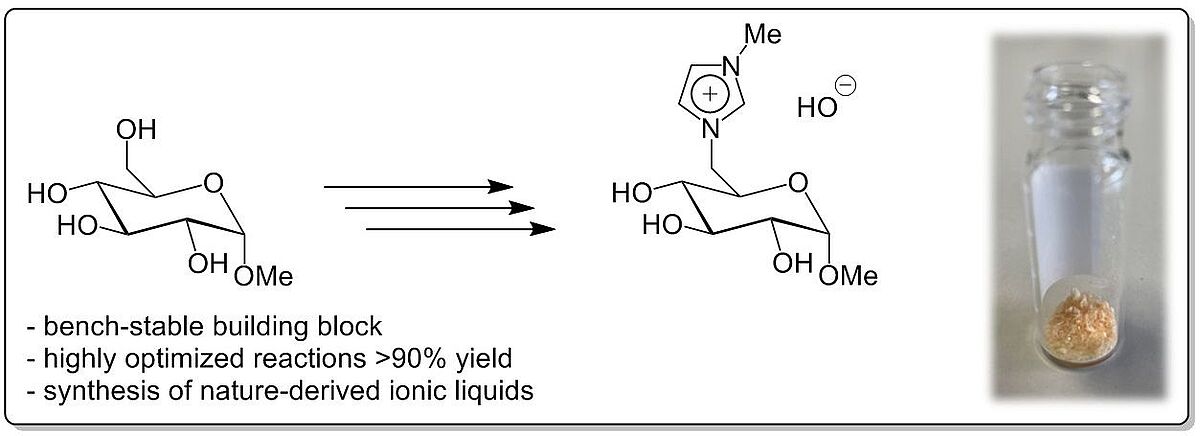
Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block (Open Access)
14. N. Tka, M. A. H. Ayed, M. B. Braiek, M. Jabli, N. Chaaben, K. Alimi, S. Jopp, P. Langer*, Beilstein J. Org. Chem. 2021,17, 1629–1640.
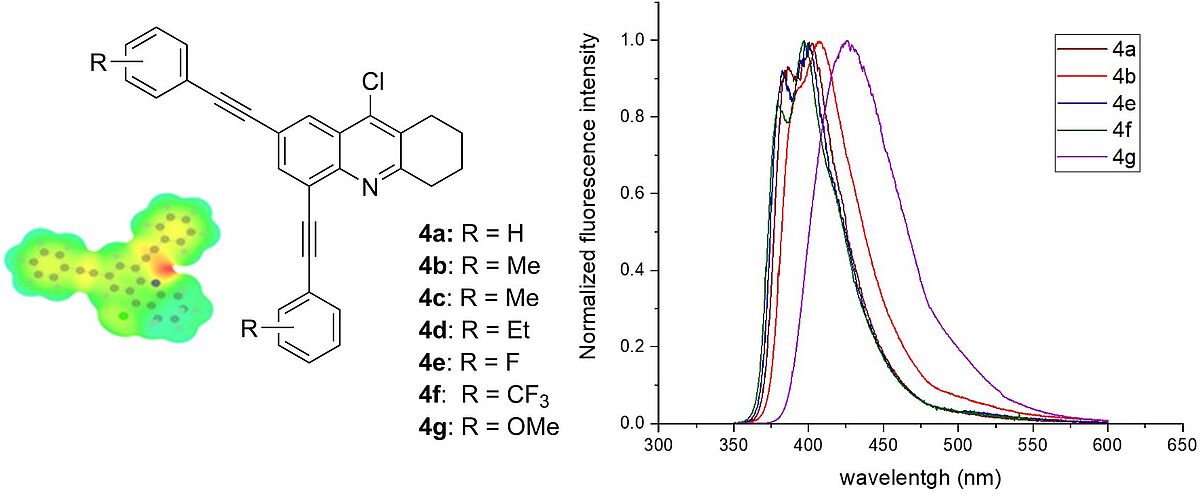
2,4-Bis(arylethynyl)-9-chloro-5,6,7,8-tetrahydroacridines: synthesis and photophysical properties (Open Access)
13. M. Komabayashi, T. Stiller, S. Jopp*, J. Mol. Liq. 2021, 325, 115167.
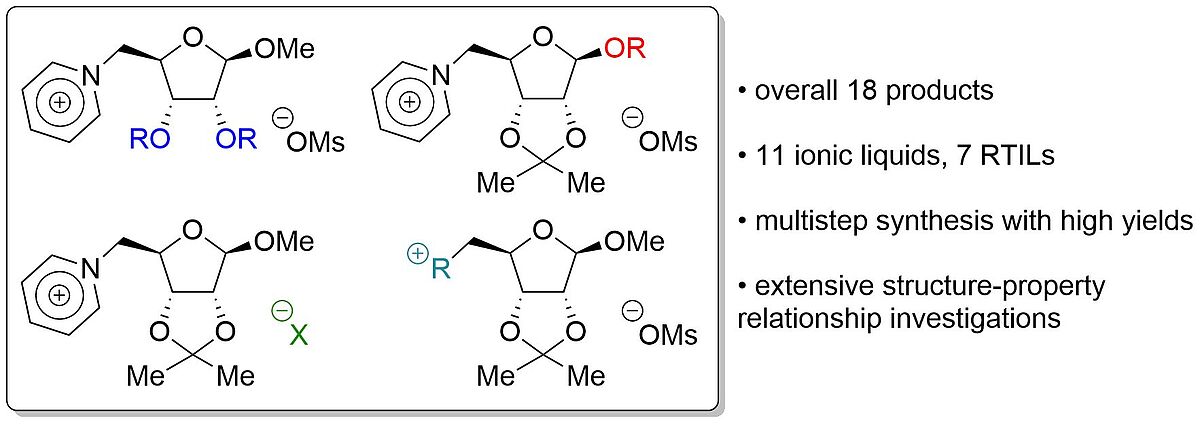
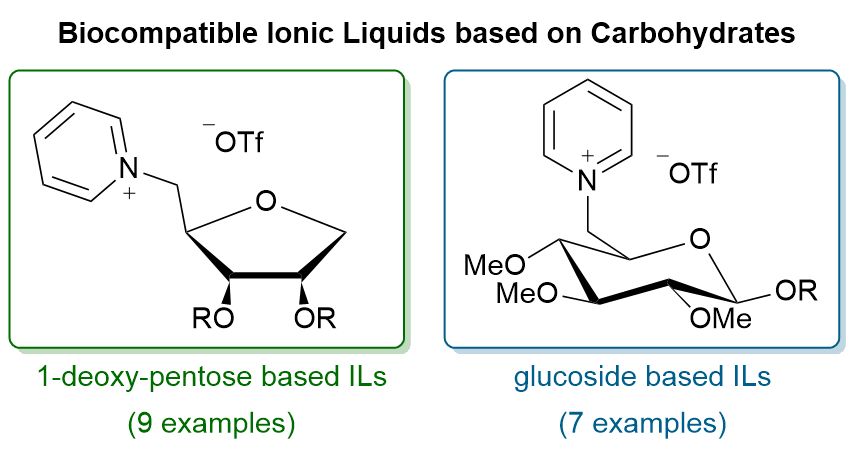
Structure-property relationships of ribose based ionic liquids
12. M. Komabayashi, T. Nokami, S. Jopp*, Asian J. Org. Chem. 2020, 9, 2092–2094.
From Chitin to CHILs: First Glucosamine based Ionic Liquids (Open Access) + Frontcover
11. S. Jopp*, Eur. J. Org. Chem. 2020, 6418–6428.
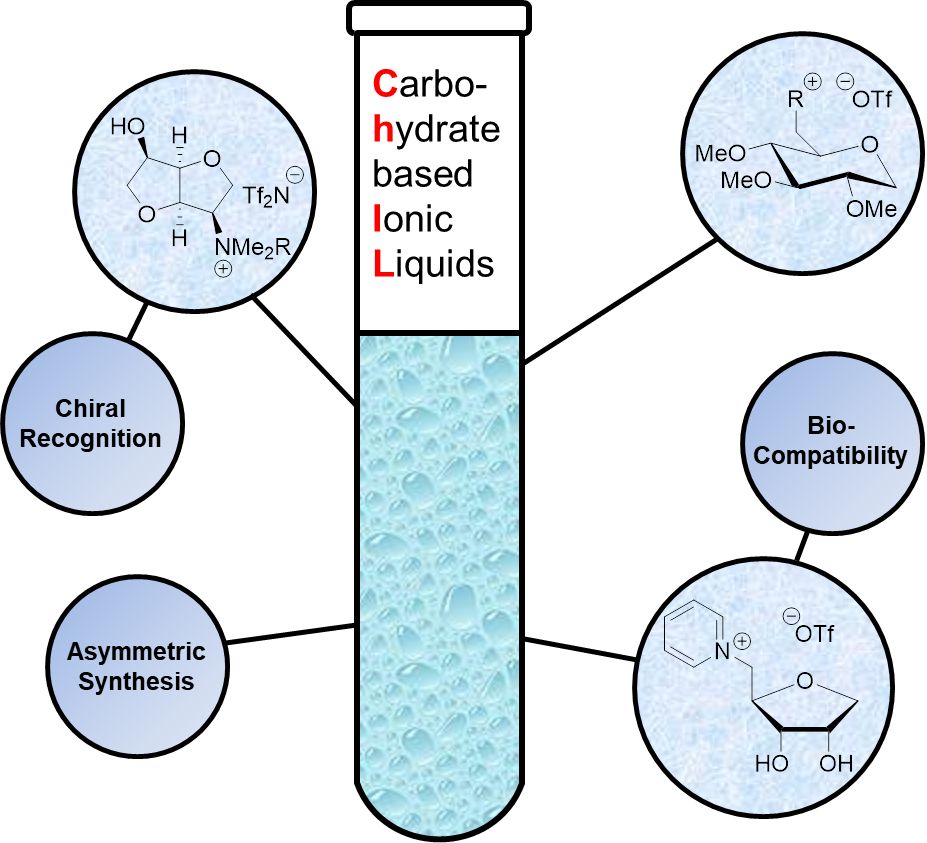
Carbohydrate Based Ionic Liquids (CHILs): Synthesis and Applications (Open Access)
10. M. Reiß, A. Brietzke, T. Eickner, F. Stein, A. Villinger, C. Vogel, U. Kragl, S. Jopp*, RSC Adv. 2020, 10, 14299–14304.
Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells (Open Access)
9. S. Jopp, R. Molenda, E. R. D. Seiler, M. F. Maitz, A. Villinger, P. Ehlers, P. Langer*, ChemistrySelect 2019, 4, 13802–13805.
Total Synthesis of Dabigatran Revisited; Synthesis of Amidine-Tosylated Dabigatran
8. L. Longwitz, S. Jopp, T. Werner*, J. Org. Chem. 2019, 84, 7863−7870.
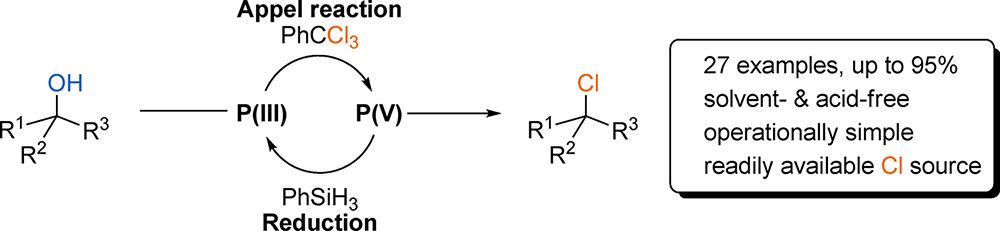
Organocatalytic Chlorination of Alcohols by P(III)/P(V) Redox Cycling
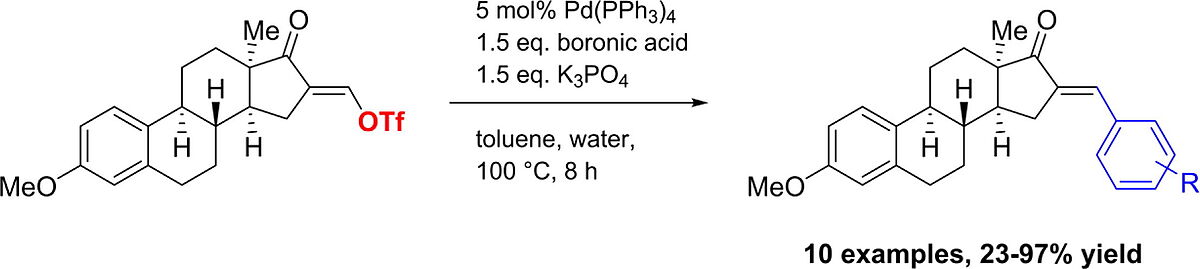
7. S. Jopp, P. Ehlers, E. Frank, E. Mernyák, G. Schneider, J. Wölfling, A. Villinger, P. Langer*, Synlett 2019, 30, 600–604.
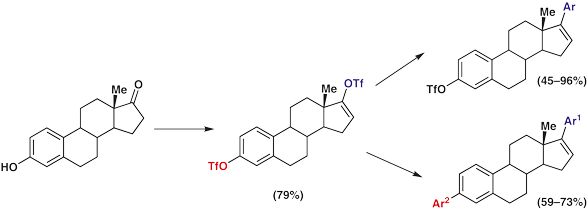
Site-Selective Synthesis of 3,17-Diaryl-1,3,5,16-estratetraenes
6. S. Jopp, T. Wallaschkowski, P. Ehlers, E. Frank, G. Schneider, J. Wölfling, E. Mernyák, A. Villinger, P. Langer*, Tetrahedron 2018, 74, 2825–2836.
5. S. Jopp, M. Liesegang, P. Ehlers, E. Frank, G. Schneider, J. Wölfling, P. Langer*, Tetrahedron Lett. 2018, 59, 26–28.
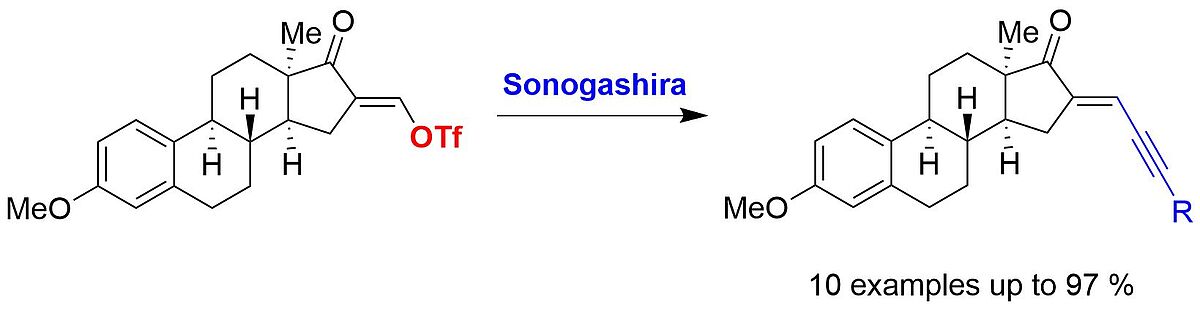
4. S. Jopp, M. Liesegang, P. Ehlers, E. Frank, G. Schneider, J. Wölfling, A. Villinger, P. Langer*, Synlett 2017, 28, 2647–2649.
Palladium-Catalysed Sonogashira Reactions of 16-(Hydroxymethylidene)-3-methoxy-α-estrone
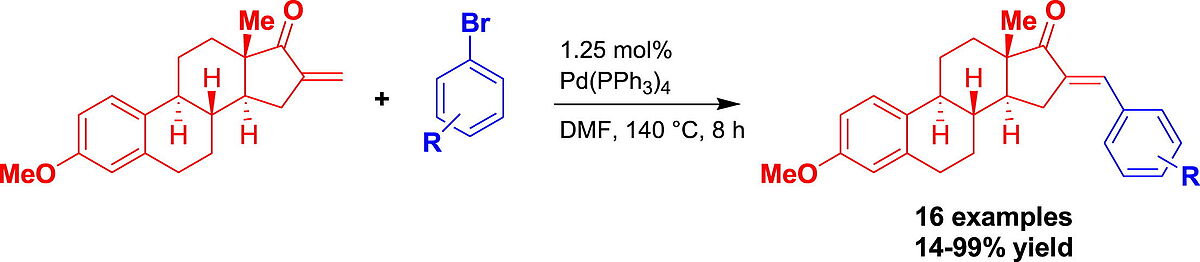
3. S. Riebe, S. Jopp, P. Ehlers, E. Frank, G. Schneider, J. Wölfling, A. Villinger, P. Langer*, Tetrahedron Lett. 2017, 58, 2801–2803.
Synthesis of 16-E-([aryl]idene)-3-methoxy-estrones by a palladium catalysed Mizoroki-Heck reaction
2. M.-L. Schirmer, S. Jopp, J. Holz, A. Spannenberg, T. Werner*, Adv. Synth. Catal. 2016, 358, 26–29.
1. P. Ehlers, A. Petrosyan, J. Baumgard, S. Jopp, N. Steinfeld, T. V. Ghochikyan, A. S. Saghyan, C. Fischer, P. Langer*, ChemCatChem 2013, 5, 2504–2511.